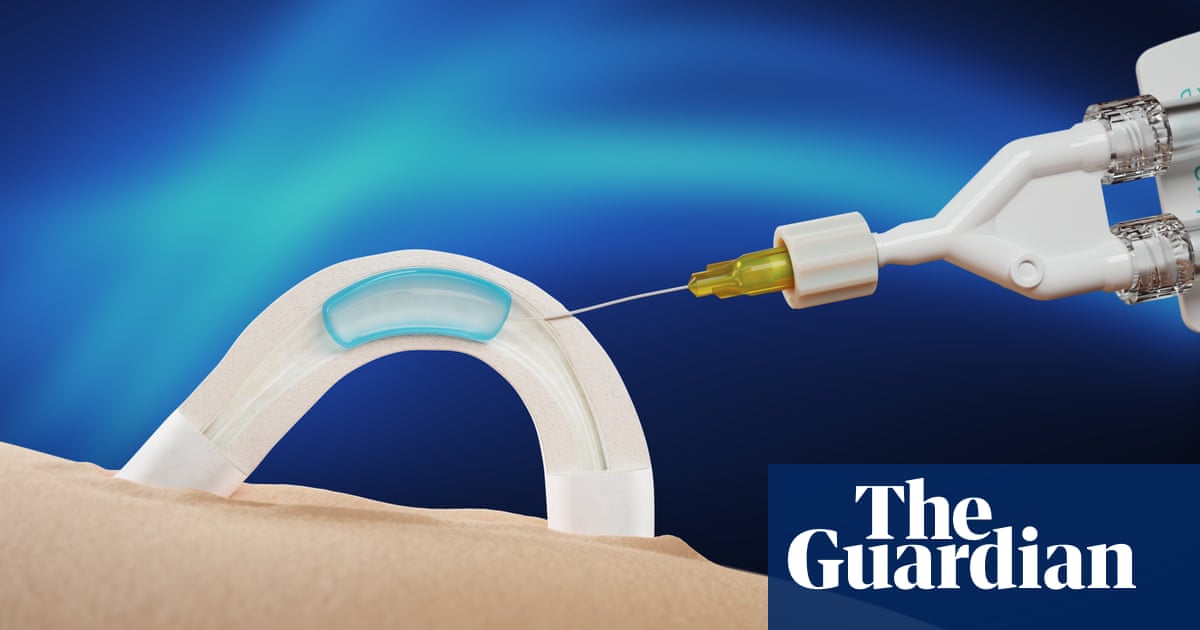
An implantable, non-hormonal male contraceptive has been shown in trials to last for at least two years.
The contraceptive, known as Adam, is a water-soluble hydrogel that is implanted in the sperm ducts, preventing sperm from mixing with semen.
The company behind the product, Contraline, says the approach offers a reversible alternative to condoms and vasectomies, with the hydrogel designed to break down in the body after a set period of time, restoring fertility.
Contraline has released details of its phase 1 clinical trial, revealing Adam can successfully block the release of sperm for 24 months, with no sperm detected in the semen of the two participants who have so far reached this time point in the trial. In addition, it said no serious adverse events had been recorded.
“This is really exciting because our goal since day one has been to create a two-year-long male contraceptive – that is what the demand is for: a two-year-long, temporary or reversible male birth control. And we have the first data to show that that’s possible,” said Kevin Eisenfrats, the founder and chief executive of Contraline.
Eisenfrats said the 25 participants in the clinical trial were enrolled at different points in time, with more results expected to follow. “It’s great proof of concept,” he added..
Eisenfrats said the implant was inserted in a minimally invasive procedure that took about 10 minutes and used local anaesthetic, meaning the patient remained awake.
Adam is not the first male contraceptive in development that acts by blocking the sperm ducts (vas deferens), although Eisenfrats said some other implants had used materials that did not break down in the body. He said there was little data to show fertility was restored after these were removed, while there were also concerns such implants could cause scarring of the vas deferens and lead to permanent sterilisation.
The results from the Adam clinical trial have not yet been published in a peer-reviewed journal and do not include data on the reversibility of the implant. However, Eisenfrats said the hydrogel had a predictable lifespan and had been shown to break down over time in animal trials, with work using lower doses in men revealing a shorter period of efficacy.
“The way to think about this is sort of like the IUD [intrauterine device] for men,” Eisenfrats said, adding that after a two-year period men could decide whether to get another implant. The team is working on a procedure to enable “on-demand reversal”. Eisenfrats said sperm tests could be used by men at home to check whether the contraceptive was still effective.
Contraline said it was expecting to begin a phase 2 clinical trial in Australia later this year involving 30 to 50 participants.
Prof Richard Anderson, an expert in hormonal male contraception at the University of Edinburgh, welcomed the findings. “It’s impressive that this looks like something that does actually work, which is great,” he said.
‘We’ve now got hormonal and non-hormonal methods in advanced clinical development, which is potentially a much better position than we’ve been in previously in terms of actually getting something on the market for men to really use.”
But Anderson and Prof Jon Oatley, of Washington State University, said at present no data had been released showing the reversibility of the Adam implant, and it remained unclear how long a single implant lasted.
Anderson also said it had yet to be shown that the implant could be removed, while Oatley said the long-term ramifications of blocking the vas deferens were unknown.
Oatley said that while the Adam implant could be a strong contraceptive option for men, uptake may be limited. “Given a choice of a pill, patch, injectable or surgery, I believe that most men would choose pill or patch over surgery,” he said.